Synopsis: Matt Finch discusses the anti-opioid vaccines being studied by the National Institutes of Health (NIH) Helping To End Addiction Long-Term (HEAL) initiative, as well as the science behind the vaccine, how it works, the ethical and logistical concerns, whether or not pharmacists think it should be mandatory, and the NIH HEAL initiative’s 6-point plan to revolutionize opioid addiction treatment, opioid overdose reversal medicines, chronic pain medications and treatment, and much more.
Sources mentioned in this episode:
- https://www.eurekalert.org/pub_releases/2021-04/uoh-urj041921.php
- https://clinicaltrials.gov/ct2/show/NCT04458545
- https://heal.nih.gov/research
- https://heal.nih.gov/about/research-plan
- https://bmcmedethics.biomedcentral.com/articles/10.1186/s12910-021-00599-2
Right-click here and save as to download this episode on your computer.
Matt Finch: An anti-opioid vaccine would protect the brain and nervous system by stimulating the body to create powerful antibodies that target and bind to opioid molecules, preventing them from crossing the blood-brain barrier to reach the brain by blocking opioids in the brain. The vaccine would reduce respiratory depression brought on by opioids when they reach the brain. Selection of an appropriate population for anti-opioid vaccine use is potentially even more fraught than the average case, as considerations of mandated use and application to vulnerable populations have been a challenge in the context of both vaccination and substance use disorder treatment.
Matt Finch: One relevant example of mandatory therapy is a vulnerable population is court-ordered medical treatment subsequent to a drug possession and distribution offense. Such policies lead to potentially coercive use of medication-assisted treatment for substance use disorders and incarcerated populations.
Announcer: Thanks for tuning in to the Elevation Recovery Podcast, your hub for addiction recovery strategies hosted by Chris Scott and Matt Finch.
Matt Finch: Hi, welcome to the show. This is Matt Finch bringing you episode 189 on a new opioid vaccine that is bringing up many ethical and logistical considerations. The opioid vaccine is a product of the National Institutes of Health, Helping End Addiction Long-term initiative, HEAL. In this episode, you're going to learn how the opioid vaccine works, when it will potentially be available, the main ethical and logistical considerations, and why opioid vaccination could become mandatory in the future.
Matt Finch: In addition to learning all about the opioid vaccines on the horizon, you're also going to learn about the other public health issues National Institutes of Health, HEAL is tackling over the next six years with literally billions and billions of dollars of funding. Including their goals of creating longer-acting buprenorphine, longer-acting methadone, longer-acting naloxone, non-opioid pain therapies, novel medication options for opioid use disorder and overdose, new strategies to prevent and treat opioid addiction, clinical research in pain management, enhanced outcomes for infants and children exposed to opioids, and last but not least, translation of research to practice for the treatment of opioid addiction.
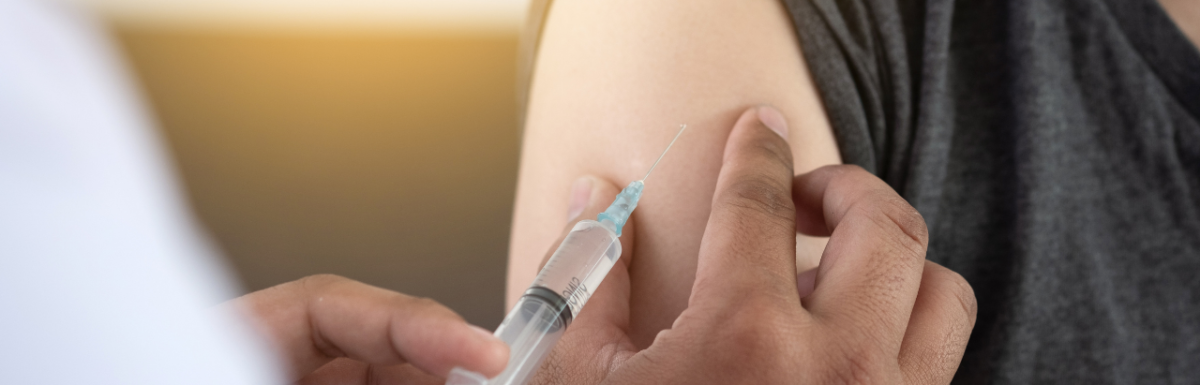
Matt Finch: Let's begin by asking the question, will the new opioid vaccine stop the epidemic? Okay, let's start with this new opioid vaccine on the horizon. According to a press release a few days ago, 19th of April 2021, "With a $25,000,000 grant from the National Institutes of Health, Helping to End Addiction Long-term initiative, HEAL, vaccine scientists from the Precision Vaccines Program at Boston Children's Hospital have partnered with Therese Kosten, University of Houston Professor of Psychology, and her colleagues Greg Cooney, Associate Professor of Medicinal Chemistry in the UH College of Pharmacy, and Dr. Colin Hale, MD Research Assistant Professor of Psychology, to develop an edge of an opioid use disorder vaccine, an adjuvant molecule boost the immune system's response to vaccines, a critical component for the effectiveness of anti-addiction vaccines."
Matt Finch: "The vaccine targets fentanyl, a synthetic and very potent opioid. An anti-opioid vaccine would protect the brain and nervous system by stimulating the body to create powerful antibodies that target and bind to opioid molecules, preventing them from crossing the blood-brain barrier to reach the brain. By blocking opioids in the brain, the vaccine would reduce respiratory depression brought on by opioids when they reach the brain. Fentanyl poses an especially difficult problem because it is often added to street drugs like cocaine, methamphetamines, and even counterfeit benzodiazepines like Xanax, which adds the amount of fentanyl overdoses."
Matt Finch: Right, so now we're going to get a little deeper into how the opioid vaccine actually works. I had to dig around and find a bunch of different articles, clinical trials, websites, and even YouTube videos to put this all together for you because it's a fascinating story and potentially very helpful.
Matt Finch: This next quote is from clinicaltrials.gov, from a page called Clinical Trials of Multivalent Opioid Vaccine Components. This was last updated February 1st, 2021. "Brief summary, currently abusive prescription opioid analgesics and heroin is a serious problem in the US, although several medications, including methadone, buprenorphine, and naltrexone are available and effective in treating opioid use disorder, OUD, long-term relapse rates remain high."
Matt Finch: "The current study is designed to examine a new approach to treating OUD, namely use of a vaccine targeted against oxycodone, one of the most commonly abused prescription opioids. The vaccination approach to treating substance use disorders relies on the ability of the vaccine to produce antibodies that bind the target drug in blood and produce its ability to enter the brain."
Matt Finch: On another article, they said it in a pretty cool way. So what the antibodies do is they bind to the opioid molecules and they actually sequester it in the blood, and then thus preventing it from crossing the blood-brain barrier and getting into central nervous system activity. So it took me a lot of reading different articles and studies to put this together because there was not one complete, coherent, fully comprehensive piece on it.
Matt Finch: A current study proposes to evaluate safety, aim one, degree of antibody production, aim two, and efficacy, i.e, ability to reduced drug liking following opioid administration, aim three. The oxycodone vaccine will be tested in participants with OUD. Target number completers equals 45 across two study sites. This study will provide a great deal of information about the safety and potential effectiveness of the oxycodone vaccine and reducing the abuse of opioids.
Matt Finch: Here's exactly how it works. Immunization will occur at weeks zero, three, six and 18. Oxycodone vaccine absorbed to aluminum adjuvant, what that word is, hydrogel or aluminum adjuvant as placebo will be injected intramuscularly, IM, into the deltoid muscle. Each subject completing the study will participate for 43 weeks, including on screening phase, two inpatient phases, two outpatient phases, and a follow-up phase.
Matt Finch: Subjects will be immunized during week zero, three, six, and 18, with either oxycodone at 100 or 400, looks like UG per dose. Different dose levels will be administered by varying the volume absorbed on aluminum adjuvant or aluminum alone as placebo. All right, for the final source, I'm going to quote, and then we're going to move past the vaccine and to some of the cool things they're researching into chronic pain, into new novel opioid use disorder and overdose drugs. But this is actually from bmcmedethics.biomedcentral.com. That's a mouthful.
Matt Finch: The title of this research article published on the 25th of March 2021 is Pharmacy Stakeholder Reports on Ethical and Logistical Considerations in Anti-Opioid Vaccine Development. "Traditionally, vaccines were designed to prevent widespread infectious diseases. However, increased interest in the use of vaccines for prophylaxis and treatment of substance use disorders have altered the way researchers are thinking about drug development and implementation. Unlike medication-assisted treatment, the general mechanistic principle of vaccine therapy involves the production of anti-drug antibodies, which upon exposure to a drug target, sequester the target molecule in the periphery, thus blunting any rewarding or adverse effects arising from their access to the central nervous system."
Matt Finch: "Pre-clinical data suggests the anti-opioid vaccines may be used as monotherapy for OUD, or theoretically in combination with the current standard of care. However, despite this favorable data, there are a variety of ethical, logistical, and clinical barriers to consider prior to implementation of anti-opioid vaccines. Pertinent questions regarding public health, society, and individuals have been raised for all vaccines belonging to the evolving class of immunotherapies designed for all types of SUD."
Matt Finch: "Systematic assessment of whether these vaccines will be supported for use in the prevention versus treatment of SUD, and investigations into opinions regarding their appropriate use and specialized populations such as children, military members, incarcerated individuals, and hospitalized patients, has been called for by medical ethicists and clinical researchers, but not yet undertaken. Given the enthusiasm for exploration of these dimensions and the paucity of current studies addressing them, this study represents an important first step and analysis of vaccine-based SUD treatments."
Matt Finch: "Furthermore, in addition to the multi-faceted ethical dilemmas pertinent to SUD vaccine approaches, logistical barriers surrounding anti-opioid vaccine deployment are likely to intersect with ethical concerns in regard to effective implementation. Such pragmatic considerations are not unlike those seen for other OUD medications, has lacked confidence and support, time and reimbursement, have been cited as critical reasons for limited use of buprenorphine and naloxone products."
Matt Finch: "Determination of which clinical population to target with a given intervention is a critically important decision during the development of a new therapeutic intervention, as it informs the design of clinical trials, directly constrains the indications considered for approval by regulatory bodies and often determines coverage access to the treatment itself. Selection of an appropriate population for anti-opioid vaccine use is potentially even more fraught than the average case, as considerations of mandated use and application to vulnerable populations have been a challenge in the context of both vaccination and substance use disorder treatment."
Matt Finch: "One relevant example of mandatory therapy as a vulnerable population is court-ordered medical treatment, subsequent to a drug possession and distribution offense. Such policies lead to potentially coercive use of medication-assisted treatment for substance use disorders in incarcerated populations. These types of programs have been studied since the late 1990s and have grown substantially in the populations they serve. Therefore, we surveyed pharmacist rated support or opposition for the mandatory and voluntary use of vaccine-based therapies across various clinical scenarios and indifferent vulnerable populations."
Matt Finch: "A global preference for voluntary use as compared to mandatory use was reported P less than 0.05 for all comparisons. Despite the wide variability in responses regarding their level of support for mandatory vaccination across multiple clinical scenarios on average, pharmacists in this sample did still exhibit support for voluntary use overall and opposition to mandatory use in most scenarios. Within this broader finding, there's a noteworthy trend that is seen in both voluntary and mandatory vaccinations scenarios. Reported support for voluntary OUD vaccination appears to increase through a general course of OUD clinical progression."
Matt Finch: "This generalized clinical course starts with the patient at risk for OUD, followed with an OUD diagnosis, eventually resulting in an opioid overdose, followed by presentation and drug court, and finally recovery. Pharmacists were increasingly supportive of voluntary OUD vaccination at each of these stages. Using the same timeline progression, pharmacists initial opposition to mandatory vaccination decreased at each stage, finally crossing over into weak support for mandatory use in drug court."
Matt Finch: "However, at the point of recovery, pharmacists favorably toward mandatory vaccination returned to opposition overall. Given this pattern of results, pharmacists' default preference for supporting patients' autonomous decision-making seemed to weaken as OUDD risks progressed throughout the course of the illness. However, once recovery is reached, autonomous decision-making is again seen as preferable and support for voluntary vaccination reaches it's maximum, perhaps indicating an expectation for self-selection for treatment from those individuals who still perceive themselves to be at high risk for negative outcomes, amongst vulnerable populations where opioid vaccination has been suggested for use, including children or adolescents at risk for OUD, pregnant women with OUD, and prisoners with OUD, the same clear preference for voluntary versus mandatory use can be seen as in when considering a general adult patient with a notably large group of respondents opposed to use of vaccination in children under either circumstance."
Matt Finch: "To our knowledge, this study represents the first attempt to get empirical knowledge about ethical concerns related to vaccine-based therapies for SUDs from relevant stakeholder populations. Given the non-infectious nature of SUDs, the ethical considerations for anti-drug abuse vaccines differ substantially than their counterparts directed against communicable diseases. The potential infringement on autonomy is often cited by opponents of vaccine-based SUD treatment technology."
Matt Finch: "With respect to this concern, pharmacists responded with a clear opposition of mandatory vaccination across clinical stages of OUD, indicating that patient awareness of such infringements may be variable over the course of SUD. Another ethical dimension of vaccine development is concerned with cost versus benefit. While pharmacists and students generally indicated that patient costs should be minimized and access should be maximized, further study regarding acceptance of specific health systems approaches to achieve these goals as required, a final concerning ethical dilemma with vaccine-based SUD treatments is the potential for vaccine administration leading the patients to use higher doses of abused drugs, or switching from one abused drug to another."
Matt Finch: "Pharmacists and students indicated a relatively low level of concern in this ethical dimension. Further studies should aim to assess other relevant stakeholders whose perspectives and experiences may allow them to better speak to the degree which vaccine-based technology may result in the promotion of risky behaviors."
Matt Finch: I'm just going to read the conclusion of this long, long article, and then we'll move on to the other things that we have to discuss today. "Conclusions, preclinical research has demonstrated that bioconjugate vaccines against small molecules can consistently block both the therapeutic and euphoric effects of drugs of abuse in rodent models. But many unresolved questions remain in regard to logistical implementation and ethical use of anti-opioid vaccines in the human population."
Matt Finch: Okay, now let's move back to the National Institutes of Health, HEAL initiative. Here's the overview. "The Helping to End Addiction Long-term initiative or NIH HEAL initiative is a trans-NIH research effort focused on improving prevention and treatment for opioid misuse and addiction and enhancing pain management. The NIH HEAL initiative is organized into six research focus areas. Within those focus areas, 12 NIH institutes and centers are leading 25 research programs to find scientific solutions to the opioid crisis helping with pain management."
Matt Finch: All right, so here are the six sections, number one, translation of research to practice for the treatment of opioid addiction. Number two, new strategies to prevent and treat opioid addiction. Number three, enhanced outcomes for infants and children exposed to opioids. Four is novel medication options for opioid use disorder and overdose. Five is clinical research and pain management, and six is pre-clinical and translational research in pain management.
Matt Finch: So we're not going to cover all of these because, within these six, there are five or six different sections in each thing. So it would literally take hours and hours and hours, but let's cover the pain stuff, pain management, and let's cover some of the things related to opioid overdose and opioid addiction. This sub-section is titled optimizing the duration, retention, and discontinuation of medication treatment for opioid use disorder. First of all, well done. I love the word optimizing.
Matt Finch: That's cool. This is great stuff. Medication treatment for opioid, use disorder, they are going to study and optimize the duration, retention, and discontinuation, very cool stuff. "The research needs patients who remain on medication treatment for opioid use disorder for a longer time, tend to have better outcomes. The risk of relapse greatly increases after patients stopped taking medication, retaining patients and treatment remains a challenge and little is known about when patients can safely stop treatment."
Matt Finch: Sounds like they just want to get people on medication-assisted treatment forever if you ask me. "This program will test strategies to improve retention and medication-based treatment for OUD, as well as strategies to improve outcomes among patients who have been stabilized on OUD medications and want to stop taking medication. The research will also identify patient characteristics associated with relapse after discontinuation and develop a predictive risk model for relapse."
Matt Finch: "The study will be a two-phase randomized clinical trial. The first phase will focus on retention and strategies to reduce dropout rates for medication-assisted treatment. The second phase will examine discontinuation, including assessing how to end medication-based treatment and the role of behavioral interventions." And here's a cool section too. This subheading is sleep dysfunction as a core feature of opioid use disorder and recovery. "Over 75% of people with opioid use disorder have sleep problems such as having irregular sleep schedules and not sleeping well or long enough. Sleep affects many mechanisms that are involved in opioid misuse, including reward and reinforcement, mood regulation, stress, risk-taking behavior, and pain perception. Sleep deficiency has many effects beyond sleepiness, including alteration of attention, emotion, memory processes at the cellular level. Getting adequate sleep reduces the ability of brain cells to function properly. It makes coping with the stress of OUD more difficult."
Matt Finch: "It is important to learn whether sleep deficiency contributes to the overuse of opioids and addiction and how individuals respond to medication treatments to overcome addiction. This program will support basic and clinical research to identify the behavioral and molecular mechanisms that directly connect sleep to the biological underpinnings of OUD. It will include research on how sleep and circadian rhythms are related to opioid addiction, withdrawal, relapse, and response to medication treatment."
Matt Finch: "More research is needed to explore these mechanisms as potential therapeutic agents and the prevention and treatment of opioid addiction. Some of the research will involve experimental models of sleep and circadian deficiency in OUD, including behavioral, pharmacological, and genetic models. Researchers will use an array of approaches such as In Vivo, In Vitro, genomic, imaging, pharmacologic, and computational strategies to study behavioral, physiological, molecular, genetic, and pharmacological mechanisms that combine sleep with OUD. This program will help researchers improve existing treatments for OUD and point the way to new therapies."
Matt Finch: The next subsection is optimizing, there's that wonderful word again. Optimizing care for people with opioid use disorder and mental health conditions. "There's an urgent need to identify approaches to treat people who have an opioid use disorder and co-occurring mental health conditions, especially in primary care settings. Data from the 2016 national survey on drug use and health suggests that among adults who misused opioids in the prior year, 42.8% also had a mental illness, and 15.6% had a serious mental illness. The collaborative care model is a promising approach to meeting the needs of people who have both OUD and mental health conditions. Collaborative care is a specific service delivery model for treating mental and behavioral conditions in primary care settings."
Matt Finch: This will be the final subheading we'll cover in this first section. It's titled prevention of progression to moderate or severe opioid use disorder. "About the program. To study the efficacy of prevention interventions to arrest the progression from risky opioid use to more severe IUD, researchers will test the efficacy of a sub-threshold opioid use disorder prevention, stop intervention in primary care settings. Risky opioid use is defined as one, having taken prescribed opioids for non-medical use or having used illicit opioids, and two, meeting no more than three of five diagnostic criteria for OUD and the diagnostic and statistical manual for mental disorders, a fifth edition. Stop adopts an early intervention approach based on a collaborative care model and consists of a practice embedded nurse care manager who provides patient education and supports the Primary Care Provider, PCP, in engaging, monitoring, and guiding patients who have risky opioid use. Brief advice delivered to patients by their PCP and phone counseling by behavioral health providers to motivate patients and support behavior change."
Matt Finch: Let's go now into one of the six sections, main sections. This one is clinical research in pain management. "Advancing clinical research on pain management is a core goal of the Helping to end Addiction Long-term initiative. The initiative will support both the new clinical trial programs and the expansion of existing programs to evaluate the safety and efficacy of innovative therapies for pain management. These clinical trials will help establish evidence-based guidelines for treating pain with non-opioid therapies to reduce use of prescription opioid medications."
Matt Finch: "Research programs, acute to chronic pain signatures program. Research from the acute to chronic pain signatures program will form a comprehensive data set that can be used to help predict which patients will recover from acute pain associated with surgery or injury, and which ones will develop long-lasting chronic pain. This information will help guide approaches to pain management."
Matt Finch: "Early phase pain investigation clinical network. The early phase pain investigation clinical network seeks to enhance the treatment of acute and chronic pain and reduce reliance on opioids by establishing a network for early phase clinical trials of non-addictive treatments for pain. EPPIC-Net is made up of more than 12 specialized clinical centers, each of which has multiple sites and two centers to support infrastructure."
Matt Finch: "Back Pain Consortium Research Program. In conjunction with the Early Phase Pain Investigation Clinical Network, the Back Pain Consortium Research Program will conduct studies to better understand common pain conditions such as chronic low back pain, improved methods to characterize pain, and develop improved diagnostic and treatment tools for pain. NIH will also support research to identify, prioritize, and test therapies based on a detailed analysis of the nature and cause of back pain in individual patients."
Matt Finch: "Pragmatic and implementation studies for the management of pain to reduce opioid prescribing. Pragmatic and implementation studies for the management of pain to reduce opioid prescribing prism program supports clinical research to integrate evidence-based interventions for pain and to the healthcare system. Studies will take place in real-world settings to determine the effectiveness of multiple therapies to address pain conditions."
Matt Finch: And the other section in regards to pain is titled Pre-clinical and Translational Research and Pain Management Treatment of Pain. This program seeks to validate novel therapeutic targets using a variety of methods and model systems. These targets can be applied to future translational research to speed up the creation of effective non-addictive medications for pain and reduce reliance on opioids, optimizing non-addictive therapies to treat pain. The NIH HEAL initiative will support pre-clinical optimization and development of safe, effective, and non-addictive small molecule and biologic therapeutics to treat pain."
Matt Finch: Some of the other sections on this one, which I won't go into just to save time, are discovery and validation of biomarkers and points and signatures for pain conditions, translating discoveries into effective devices to treat pain. Oh, we'll talk about that one. "The NIH HEAL Initiative will advance device-based treatments for people who currently have no effective ways to manage their pain. Research will support target identification, late-stage translational therapeutic and diagnostic device development, verification and validation activities, and early clinical studies."
Matt Finch: Moving on to another one of the main six sections. This one is called novel medication options for opioid use disorder and overdose. There are two sections and we are going to cover both of these. First one we'll do, focusing medication development to prevent and treat opioid use disorder and overdose. "The research need three medications, methadone, buprenorphine, and naltrexone, are approved by the US Food and Drug Administration for the treatment of opioid use disorder and low fluoxetine is approved to treat opioid withdrawal."
Matt Finch: "Naloxone can effectively reverse opioid overdose, but reversing respiratory arrest caused by drug combinations or powerful synthetic opioids, can require multiple doses. More flexible treatment options for OUD are needed to help more people achieve long-term recovery. About the program. The HEAL initiative is supporting a series of targeted studies with the goal of submitting approximately 15 investigational new drugs and five new drug applications to the FDA for medications to prevent and treat OUD and overdose. The program aims to accelerate the discovery and development of novel medications to treat all aspects of the opioid addiction cycle, including progression to chronic use, withdrawal symptoms, craving, relapse, and overdose."
Matt Finch: "Specifically, this program will include new formulations of existing medications, stronger, longer duration formulations to counteract opioid overdose, interventions for respiratory depression, novel medications to treat withdrawal, craving progression, and relapse, new medication targets to treat OUD. Research through this award will investigate the optimization of non-opioid medications, combinations of opioid and non-opioid medications, and new technology for the controlled release of opioids to treat pain with a lower risk of addiction."
Matt Finch: "To improve adherence to medication-based therapy, to treat OUD, researchers also plan to develop new medications and treatment regimens with increased efficacy, fewer side effects, and fewer visits to a healthcare facility. Additionally, researchers will develop therapies for treating OUD during pregnancy and for treating opioid withdrawal in newborns. Those efforts will lower long-term relapse rates and improve the quality of life of people recovering from OUD."
Matt Finch: "NIH is also assessing novel therapeutic agents through existing community-based clinical partnerships developed by the District of Columbia Partnership for AIDS Progress, a collaboration among NIH, the DC Department of Health, and the George Washington University School of Public Health. Examples of research that awardees will conduct include optimizing novel targeted non-addictive medications, and non-pharmacological treatments for acute and chronic pain, developing technology to control the release of opioid medications in the body to lower the risk of addiction, developing medications to manage withdrawal, reduce cravings, and lower the risk of a relapse in people receiving treatment for OUD."
Matt Finch: "Examples of research that awardees will conduct include optimizing novel targeted non-addictive medications, and non-pharmacological treatments for acute and chronic pain, developing technology to control the release of opioid medications in the body to lower the risk of addiction, developing medications to manage opioid withdrawal, reduce cravings, and lower the risk of a relapse in people receiving treatment for OUD, developing opioid vaccines to reduce the risk of an opioid overdose and to treat OUD, optimizing oral, injectable and implantable long-acting medication to treat OUD. Developing medication to treat insomnia in people who are receiving treatment for OUD, developing medical devices to detect an opioid overdose and automatically administer life-saving medications."
Matt Finch: Okay, we're going to start to wrap it up here. "Collaborations and governance. The NIH HEAL Initiative will forge collaborations among research programs across NIH, the US Department of Health and Human Services, and the private sector. An executive committee of NIH leaders will coordinate NIH HEAL initiative efforts to develop safe non-addictive pain medications, and other strategies for pain management."
Matt Finch: Now I'm going to quote the last couple of sections on this very, very long NIH HEAL initiative research opportunities page. "To ensure a steady and strong communication process between the public and private sector groups involved in the initiative, NIH plans to establish a consortium of NIH, FDA, and academic leaders to provide input to NIH on the overall progress of the partnership. All funding decisions will be made based on objective review of data and assets by NIH Institute directors and the relevant areas of research, with guidance from the appropriate NIH Institute advisory councils."
Matt Finch: "Federal partners. NIH will partner with the FDA, Centers For Disease Control and Prevention, Health Resources and Services Administration, Agency for Healthcare Research and Quality, Centers for Medicare and Medicaid Services, Department of Veterans Affairs, Department of Justice, and the Office of Assistant Secretary for Health, and other Federal partners, to execute the initiative through the creation of an NIH HEAL initiative, Federal partners work group."
Matt Finch: Now let's talk about deliverables and the timeline. So in the short term, they expect to, within the first three to five years, deliver a bunch of awesome results for all the chronic pain strategies, everything about chronic pain. And the longer-term, over five years, that's when they're going to be delivering pharmaceutical programs, leading to 15 investigational new drug applications and investigational device exemptions, with the goal of five new drug applications or 510K pre-market approvals for devices submitted to the FDA.
Matt Finch: So there you have it. Now you've been well-educated and informed on the new opioid vaccine that is probably going to come out in the near future. And as well as what the NIH HEAL initiative is working on, what they expect to deliver over the next few years with chronic pain advancements. And then the next few years after that, opioid use disorder and overdose and prevention advancement, opioid vaccine, or anti-opioid vaccine that is on the horizon.
Matt Finch: You've learned about this study that's going to start with the oxycodone vaccine and vaccines for fentanyl and longer-acting opioids, longer-acting opioid agonists and antagonists actually, more powerful medicines and non-opioids for pain, and so many different optimizations, it's crazy. It all sounds very wonderful. It sounds all very wonderful. It all sounds very in line with making big pharma just way, way, way... more and more and more profits. The pharmaceutical companies that are creating these new novel opioids and agonists and antagonists and opioid vaccines, they're going to be the benefactors of these initiatives.
Matt Finch: So take it all with a grain of salt. I think the more helpful medicines that we have that are well-studied, the better. I also do see, I can see how some people would think that, "Wait. Well, it doesn't look like they're actually curing chronic pain or curing people's OUD. It looks like they're treating symptoms with just better and better medications that are easier to stay on long-term and perhaps less addictive."
Matt Finch: So it's all good, we want lots of great options out there. So it looks like the twindemic of COVID and addiction and overdose together, really kicked things into the high gear. It looks like they have the money for it and how they have a really detailed six-pronged plan with a lot of different bullet points to optimize the treatment world, looks like they're really going after it.
Matt Finch: One thing I will say though, is I am very, very glad that Chris and I have this podcast because the Federal partners, NIH HEAL initiative, they can work on developing new medications, that is awesome. I can learn about those medications and they can be more resources that I have to educate people on, to where they could possibly incorporate something like that into their treatment plan if they wanted to.
Matt Finch: But I'm really glad that we have this podcast where we can educate people on many of the natural things they can do, as well as how to optimize their psychology, their spirituality, their social relationships, their physical environment, their goal setting, and all those other life areas. So the life optimization, what the foundation of biochemical optimization. In addition to having all these new upcoming novel opioid drugs that are going to be longer acting and better for reversing overdoses and preventing, getting high from opioids with even things like opioid vaccines.
Matt Finch: I am fascinated by science like this and drug pharmacology, and I think it's great. Of course, it's the government, it's got the FDA involved. They're studying medications, that's where all the money's at. The money is in treating symptoms with medications for long-term or life.
Matt Finch: So that's what they do, and what Chris and I do is a little bit different, but you need everything. You need all these types of resources. So I think this is very exciting stuff. It's by far the most impressive plan I've ever seen drawn up to help the general masses. It's really about saving lives, it's about the overdose is so high. So with the state of America right now, our poor country, with the state of the people and the addiction rates have gone up, the mental health rates have gone up, the suicide rates have gone up, the chronic pain rates have gone up. The stress has gone up, the day drinking has gone up.
Matt Finch: There is a percentage that one in six adults let their teenage kids drink alcohol during the pandemic to relieve stress. So it's harsh times right now, and this is an initiative that is going to, for sure, save a lot of lives. And there have a lot of force, they got a lot of people involved in agencies. And so, it's a lot of medications, it's going to be great.
Matt Finch: I'll continue to educate you on those as well as natural things you can do, psychology, social, environmental, domains of addiction and recovery, transcending addiction. I just love to learn about all these different resources and therapies. I love innovation, and I love seeing people take an approach to this, that it is a health crisis. It's not just this huge moral failing of all these different, millions and millions and millions of people. It's a huge health epidemic.
Matt Finch: These substances are changing people's brains, that is depleting, eroding the function of their prefrontal cortex, while simultaneously hijacking their midbrain and destroying their dopamine pleasure system and more too, and more, neurotransmitter deficiencies. It hijacks free well so much, and the more severe an addiction, the more it hijacks free will. Take someone to understand how their actions, those things that happen in the brain, to understand that it does reduce choice.
Matt Finch: So thanks so much for listening, really had fun studying and researching this topic and delivering it to you. And I'll see you next time.
Sign up to receive email updates
Enter your name and email address below and I'll send you periodic updates about the podcast.



Wow! What incredibly exciting news. This is the first I have heard of it, so thank you for all your hard work to make sure we keep informed! God Bless.
Glad you enjoyed this info!! You’re most welcome 🙂
I rather that than the covid vaccine. I would take it in a heartbeat..
how will this affect methadone treatment i have been a patients for 12 years and it has saved my life i also suffer from chronic low back pain which is severe and the methadone also controls my pain i was a heroine addict long before i hurt my back and narcotics are the only relief i have been able to find also i have been sober or clean from all other drugs besides the methadone i am perscribed for the whole 12 years so this program does work if people use it correctly so i am wondering what will happen if the vaccine only works like the suboxone cause that does not help my pain without the methadone i will have no quality of life at all so stop and think about everyones individual situation before you advocate total abstinance
I like to keep informed on what and how if this will be away to get of of methdone and not have to pains and big symptoms of withdrawal. I will over 60 and have been on methadone for year thur pain management but by government there cut back and I tried of taken this drug and always wondering when I’ll get cut off or if can’t get to next appointment. I’m ready been ready but getting handled to old ways of getting of have been hoping to find a easier way on body to be off of it all. Thanks for this information that there maybe a easier way on body
So its not really a vaccine? It sounds more like another type of blocker. They’d be better finding something that stops any withdrawals. If detoxing was easier then there would be far less relapses. The current meds, suboxone etc, can be a horrible experience. In the UK we get stuck on methadone and then after methadone we get put onto suboxone which can make you extremely ill. I recently went through the worst withdrawals I’ve ever been through because of this. These maintenance and titration meds really need to be discarded and a new, pain free medication found.
Yes please, 11 years of hell, when will it be available in the UK Matt, or I will fly to America, asap.!!!!
Why can they get some kind of medication that is not addictive so we can detox off narcotics that’s what they should be trying to accomplish. Something that’s not addictive.